Water in an estuary has dissolved salt within it. The salinity gradient generally increases from the input source of an estuary, usually a stream or river, to the output source, the sea or ocean. Salinity is measured in gravimetrically as parts per thousand of solids in liquid or ppt. The salinity of the ocean is generally around 35 ppt (Antonov 2006). Another salinity unit is the practical salinity unit or PSU measurement, which is based on water temperature and conductivity measurements made by sondes and the ocean is also generally around 35 PSU (Antonov 2006). Using ppt or PSU gives similar results for the ocean’s seawater salt content (Antonov 2006).
The fresh water from rivers has salinity levels of 0.5 ppt or less. Within the estuary, salinity levels are referred to as oligohaline (0.5-5.0 ppt), mesohaline (5.0-18.0 ppt), or polyhaline (18.0 to 30.0 ppt) (Montagna et al. 2013). Near the connection with the open sea, estuarine waters may be euhaline, where salinity levels are the same as the ocean at more than 30.0 ppt (Mitsch and Gosselink 1986). An example of this is shown in the illustration below:
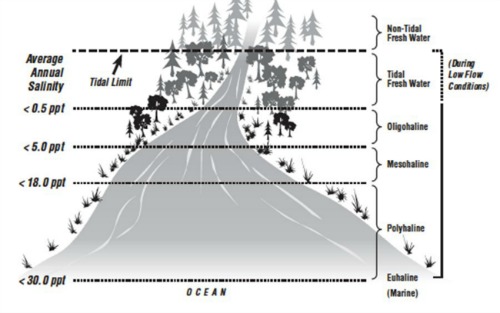
The salinity of an estuary can vary dependent on the amount of freshwater inflows as well as the tidal movement and location within the estuary. Estuaries have a water balance that is either positive, freshwater inputs exceed evaporation; neutral, there is a balance between freshwater inflows and evaporation; or negative, freshwater inflows are less than the amount of evaporation (Montagna et al. 2013).
Seasonally in the U.S., estuaries generally decrease in salinity in the spring months with increased inflows making the system a positive estuarine system. In the summer, estuaries increase in salinity with decreased freshwater inflows and increased evaporation due to higher temperatures causing the system to be classified as a negative estuarine system.
Salinity affects the chemical conditions within an estuary, most notably the amount of dissolved oxygen. Solubility is the amount of oxygen that can dissolve in water, which decreases as salinity increases. Solubility is important because estuarine organisms have salinity ranges they can tolerate before they experience stress (Montagna et al. 2013). Some estuarine species can adapt to changes oxygen levels by practicing avoidance techniques. Fish can swim away to areas with tolerable salinity levels. Other organisms such as mollusks, oysters, and benthic organisms cannot travel large distances. Salinity levels outside of their tolerance ranges cause negative affects including increased stress and decreased reproduction and survival rates (Palmer et al. 2008).